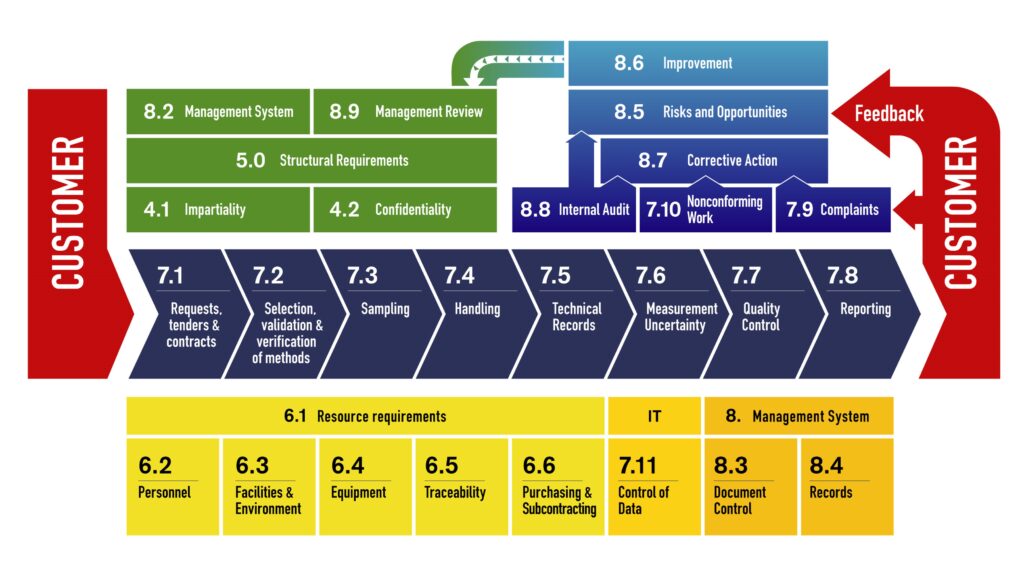
The most popular standard for the competence of testing and calibration laboratories has just been updated, taking into account the latest changes in laboratory environment and work practices. Producing valid results that are widely trusted is at the heart of laboratory activities. ISO/IEC 17025:2017 allows laboratories to implement a sound quality system and demonstrate that they are technically competent and able to produce valid and reliable results.

- The scope has been revised to cover testing, calibration and sampling associated with subsequent calibration and testing.
- The process approach now matches that of newer standards such as ISO 9001 (quality management), ISO 15189 (quality of medical laboratories) and ISO/IEC 17021-1 (requirements for audit and certification bodies).
- The standard has now a stronger focus on information technologies and incorporates the use of computer systems, electronic records and the production of electronic results and reports.
- A new chapter introduces the concept of risk-based thinking.
*Source from www.iso.org